Ezetimibe is a lipid-lowering medication that acts by inhibiting the absorption of cholesterol in the small intestine.1 The 2018 American Heart Association and American College of Cardiology Guidelines for the Management of Blood Cholesterol recommend its use along with a statin for certain high-risk populations, when statin therapy alone is insufficient to reach lipid-lowering goals.2
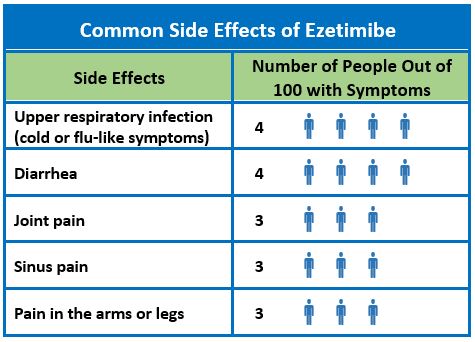
In the ezetimibe clinical trials database, side effects that occurred in more than 2% of patients taking ezetimibe monotherapy were upper respiratory infection (4.3%), diarrhea (4.1%), arthralgia (3.0%), sinusitis (2.8%), and extremity pain (2.7%).1 Creatinine kinase (CK) levels exceeded 10 times the upper limit of normal in 0.2% of patients on ezetimibe alone, compared to 0.1% in those on placebo, 0.1 % in patients taking ezetimibe and a statin, and 0.4% of patients taking a statin alone. Other side effects that have been reported with ezetimibe (frequency unknown) include:
- hypersensitivity reactions, including anaphylaxis, angioedema, rash, and urticaria
- erythema multiforme
- myalgia
- elevated creatine phosphokinase
- myopathy/rhabdomyolysis
- elevations in liver transaminases
- hepatitis
- abdominal pain
- thrombocytopenia
- pancreatitis
- nausea
- dizziness
- paresthesia
- depression
- headache
- cholelithiasis
- cholecystitis
Cannon et al. conducted a double-blind randomized trial of 18,144 patients with a history of acute coronary syndrome and elevated low-density lipoprotein (LDL) levels to evaluate the effect on cardiovascular outcomes when ezetimibe was added to statin therapy.3 Participants in the study were randomized to receive a combination therapy of ezetimibe 10 mg/simvastatin 40 mg or simvastatin 40 mg plus placebo. Safety profiles after treatment between the two groups were not significantly different with respect to elevated alanine aminotransferase levels, gallbladder-related adverse events, cholecystectomy, muscle-related adverse events, or cancer.
Kashani et al. conducted a systematic review of randomized controlled trials to assess the risks associated with combination ezetimibe and statin therapy (18 trials, n=14,471).4 There was no statistically significant difference in the risk of developing myalgias with combination therapy versus statin monotherapy. The relative risk of developing myalgias with combination therapy was 0.86 (95% confidence interval [0.60 – 1.24]) compared with statin monotherapy. Compared to statin monotherapy, there was no significant increase in other adverse events (CK increases, rhabdomyolysis, transaminase increases, or gastrointestinal adverse events) with combination ezetimibe plus statin therapy.
References
- Ezetimibe [package insert]. Whitehouse Station , NJ: Merck & Co, Inc; 2007.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol. J Am Coll Cardiol 2018.
- Cannon C, Blazing M, Giugliano R et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372 (25): 2387-2397.
- Kashani A, Sallam T, Bheemreddy S, Mann DL, Wang Y, Foody JM. Review of side-effect profile of combination ezetimibe and statin therapy in randomized clinical trials. Am J Cardiol 2008; 101 (11): 1606-1613.